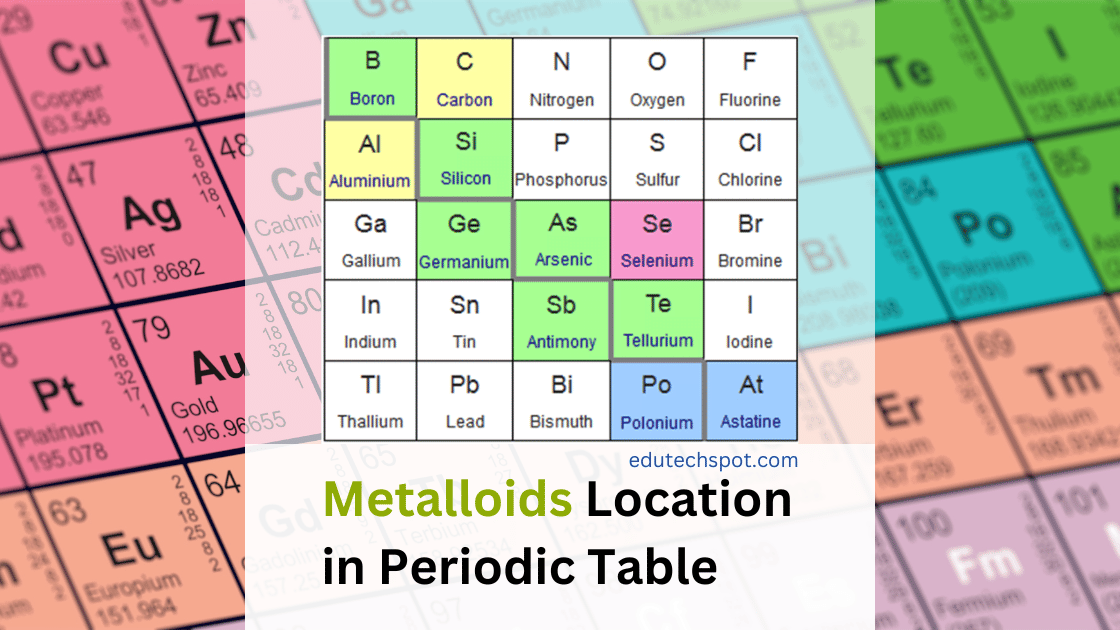
Metalloids are located where on the periodic table? Metalloids are a group of elements located on the right side of the periodic table in the p-block. They exhibit properties of both metals and non-metals, making them unique. The six commonly recognized metalloids are Boron, Silicon, Germanium, Arsenic, Antimony, and Tellurium, with Polonium and Astatine sometimes considered as well. Metalloids typically have a metallic appearance and are fair conductors of electricity. However, they chemically behave as non-metals. Let’s explore more about these intriguing elements and their properties.
Read More: 12 FREE Printable Periodic Tables PDF, PNG, SVG HD
Are you intrigued by the intriguing elements known as metalloids? These elements occupy a unique position on the periodic table, showcasing properties of both metals and non-metals. In this blog post, we will delve into the world of metalloids, exploring their location on the periodic table, commonly recognized metalloids, and their fascinating characteristics. Join us as we unveil the secrets of these enigmatic elements and shed light on their unique properties.
The Location of Metalloids on the Periodic Table:
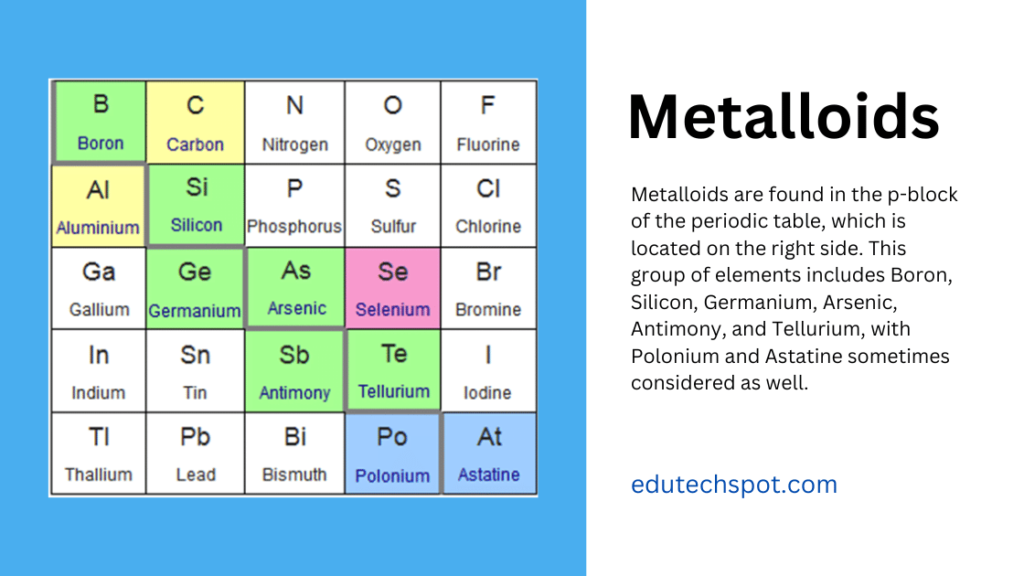
Metalloids are found in the p-block of the periodic table, which is located on the right side. This group of elements includes Boron, Silicon, Germanium, Arsenic, Antimony, and Tellurium, with Polonium and Astatine sometimes considered as well. These elements occupy a distinctive position, sharing characteristics of both metals and non-metals.
Commonly Recognized Metalloids:
There are six commonly recognized metalloids on the periodic table. Let’s take a closer look at each one of them:
- Boron (B): Boron is a metalloid that is often used in the production of heat-resistant glass and ceramics. It also has important applications in agriculture as a micronutrient for plant growth.
- Silicon (Si): Silicon is a widely used metalloid in various industries, especially in the production of semiconductors, computer chips, and solar cells. It is known for its ability to conduct electricity under certain conditions.
- Germanium (Ge): Germanium is another metalloid that is used in the production of semiconductors and optical fibers. It is also used in some medical imaging devices and as a catalyst in some chemical reactions.
- Arsenic (As): Arsenic is a well-known toxic metalloid, but it also has some industrial applications, such as in the production of insecticides, wood preservatives, and glass.
- Antimony (Sb): Antimony is a metalloid that is used in the production of flame retardants, batteries, and some medicines. It is also used in the manufacture of certain types of glass and ceramics.
- Tellurium (Te): Tellurium is a rare metalloid that is used in the production of solar cells, thermoelectric devices, and some types of glass.
Polonium (Po) and Astatine (At) are also sometimes considered as metalloids, but they are highly radioactive and have limited practical applications.
Unique Characteristics of Metalloids:
Metalloids exhibit properties of both metals and non-metals, making them distinct from other elements on the periodic table. Here are some unique characteristics of metalloids:
- Metallic appearance: Metalloids often have a metallic appearance, resembling metals in their shiny and lustrous appearance.
- Conductivity: Metalloids are fair conductors of electricity, although their conductivity may vary depending on the conditions and the specific element.
- Chemical behavior: Metalloids chemically behave as non-metals, exhibiting characteristics such as forming covalent bonds, having high electronegativity, and showing non-metallic reactions.
Metalloids are a fascinating group of elements that possess unique properties on the periodic table. They occupy a distinctive position, exhibiting characteristics of both metals and non-metals. The six commonly recognized metalloids, namely Boron, Silicon, Germanium, Arsenic, Antimony, and Tellurium, along with Polonium and Astatine, are known for their diverse applications in various industries, from electronics to glass manufacturing. Understanding the location of metalloids on the periodic table and their distinct characteristics can provide insights into their unique properties and potential applications.
Teacher has to guide students to identify Metalloids
As a teacher, it’s important to equip our students with a solid foundation in chemistry and the periodic table. One fascinating aspect of the periodic table is the group of elements known as metalloids. These elements exhibit properties of both metals and non-metals, making them unique and interesting to study. Here are some tips on how to identify metalloids:
- Location on the periodic table: Metalloids are typically found in the “staircase” region of the periodic table, which separates metals from non-metals. This includes elements such as Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), and Tellurium (Te).
- Physical appearance: Metalloids often have a metallic appearance, but may not be as lustrous or malleable as metals. They can also have properties of non-metals, such as being brittle or having lower melting and boiling points.
- Electrical conductivity: Metalloids are fair conductors of electricity, but their conductivity can vary depending on the element and conditions. They may exhibit semi-conductive behavior, making them important in the field of electronics.
- Chemical behavior: Metalloids typically exhibit intermediate chemical properties between metals and non-metals. They can form both metallic and covalent bonds, and their reactivity may vary depending on the specific element.
- Other properties: Metalloids may also have unique properties such as being used in glass manufacturing (e.g. Tellurium), as a semiconductor in electronic devices (e.g. Silicon), or as a dopant in the production of semiconductor materials (e.g. Boron).
identifying metalloids involves understanding their location on the periodic table, their physical appearance, electrical conductivity, chemical behavior, and unique properties. By familiarizing themselves with these characteristics, students can gain a deeper understanding of the intriguing world of metalloids and their significance in various applications. Happy exploring and discovering the wonders of chemistry and the periodic table!
How are metalloids different from metals?
Metalloids are elements that exhibit properties of both metals and non-metals, making them unique in the periodic table. Here are some key differences between metalloids and metals:
- Electrical conductivity: Metals are generally good conductors of electricity, whereas metalloids have intermediate conductivity. Metalloids can exhibit semi-conductive behavior, which makes them important in electronic applications.
- Physical properties: Metals are typically lustrous, malleable, and ductile, whereas metalloids may have a metallic appearance but may not be as shiny or malleable. Metalloids can also have properties of non-metals, such as being brittle.
- Melting and boiling points: Metals generally have high melting and boiling points, while metalloids may have lower melting and boiling points compared to metals.
- Chemical behavior: Metals tend to form cations (positive ions) in chemical reactions, while non-metals tend to form anions (negative ions). Metalloids can exhibit both metallic and covalent bonding behavior, depending on the specific element and conditions.
- Reactivity: Metals are generally highly reactive, while metalloids can exhibit varying levels of reactivity depending on the specific element and environmental conditions.
- Applications: Metals are commonly used in various industrial and commercial applications due to their high conductivity, malleability, and other properties. Metalloids, on the other hand, have unique applications such as in the production of semiconductor materials, glass manufacturing, and other specialized uses.
Metalloids differ from metals in terms of their electrical conductivity, physical properties, melting and boiling points, chemical behavior, reactivity, and applications. Metalloids exhibit properties of both metals and non-metals, making them unique and interesting elements in the periodic table.
Related to Metalloids are located where on the periodic table
Lesson Plan Template Google Docs
Art Drawing Websites
Free Flash Card Template for Word
Index Card Template Google Docs
Index Card Sizes